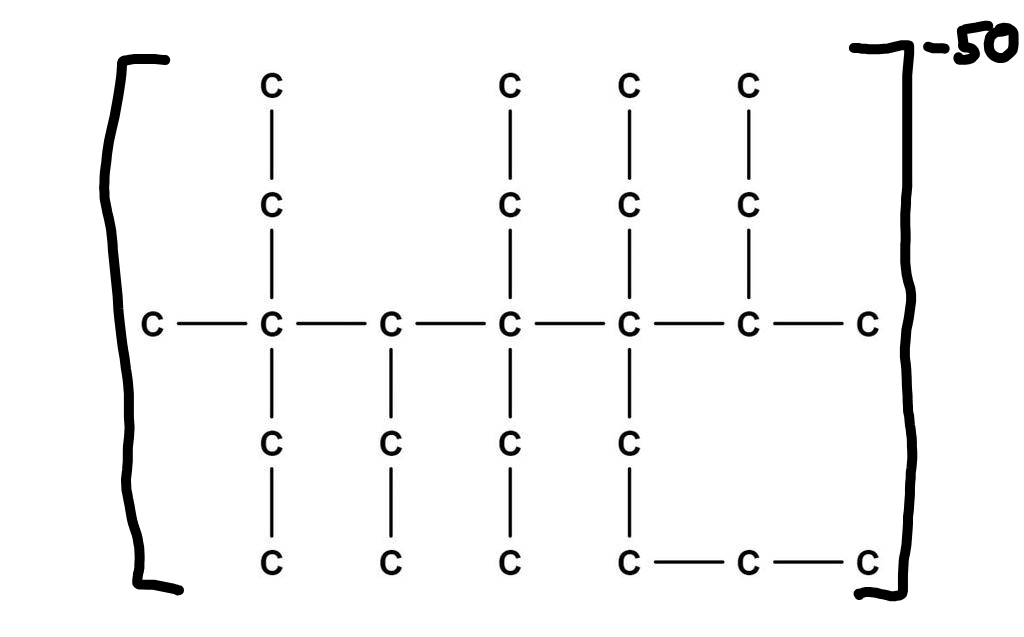
Fixed the charge on your 3-methyl-3,4,5,5,6-pentaethyl-6-butan-2-yl decane ion, aka lossane.
(It’s been a while since I last did chemistry, so apologies if I messed up the nomenclature a bit)
As someone who paid enough attention in highschool chemistry to get a B, and occasionally watches Nile(red/blue) and E&I videos… I know some of these words/symbols!
Oh by fucking gods. It’s loss. People are STILL posting loss. And here I was thinking this was a chemistry meme.
*Lossane
So … What does this chemical make you lose?
Sanity
Your marbles.
You lose nothing, don’t worry about it.
On the other hand, you learn C (the programming language) by ingesting it, which would be considered a punishment by some.
It makes you lose your way in a vast endless c
Couldn’t resist. In a more standard form this molecule should (I believe—chemistry has been a long time ago) look like this:
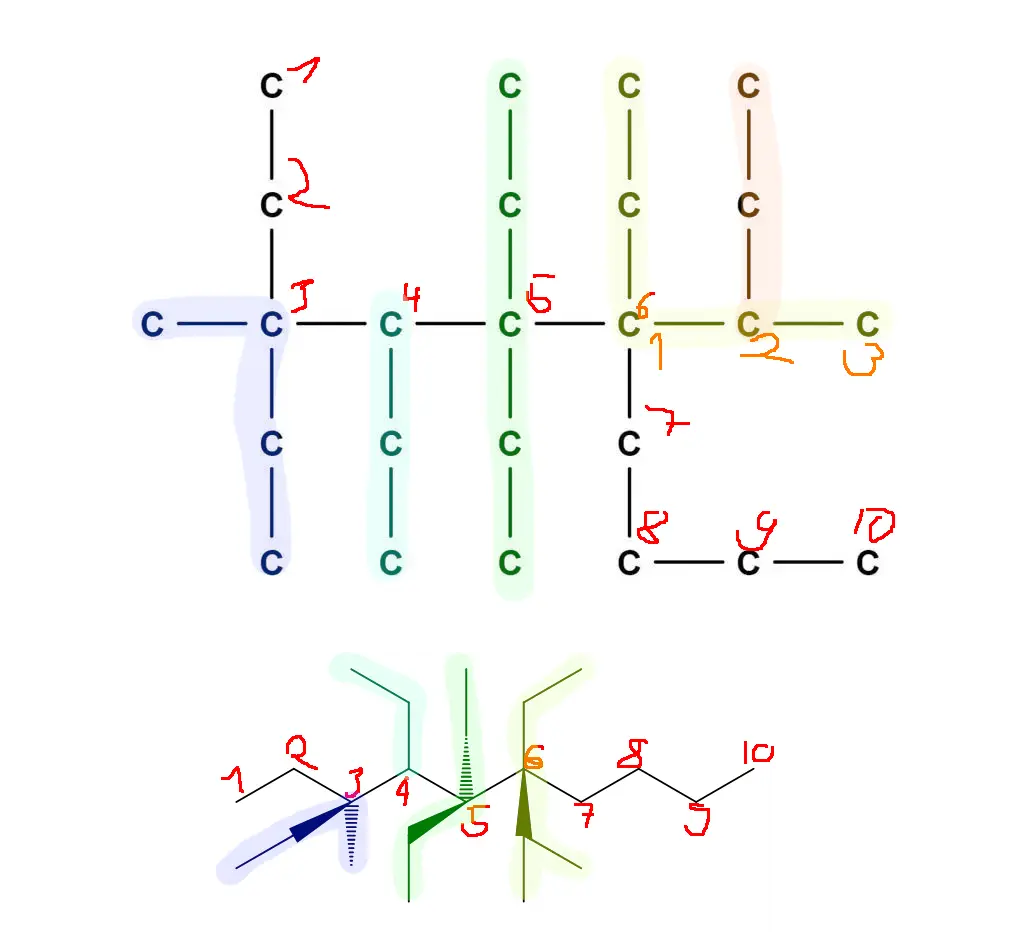
Made with MolView –https://molview.org/?smiles=C([C%40](CC)(C)C(CC)[C%40](CC)(CC)[C%40%40](C(C)C)(CC)CCCC)C
People knowledgeable in chemistry, please correct!
If I ever teach chemistry to kids imma tell them to name this
(1,2,2,50)-loss-quinquagintinane
The urge to add hydrogens to all the carbons not fully bonded is overwhelming
Assuming this has a 47 hydrogens stuck on to make it stable, I’d call it:
3-methyl-3,4,5,5,6-pentaethyl-6-buta-2-yl dodecane
dodecane has a 12 carbon chain. The longest chain here is 10 carbons, which would be decane.
I am a total chem nitwit, would you like to explain me how you come to this ?
My stoned ass thought this was a shifter tree diagram of a goofy little manual transmission for a sec. All gears are just ‘crash’, lol
I somehow recognized it immediately. I think this meme has rewritten my brain.
Ah ok so it’s Loss got it
I’m at a loss
Can someone explain this to me? I don’t get it, but I want to.
3-metilhexa-4,5,5,6,7,7-etil-4-butil From the head, im feel happy now
OK how long until it kicks in?
a few years and then you die of cancer